The Phenotype Switching Model
Metastatic potential in melanoma (or in any cancer) is a combination of invasion and proliferation. Our research has led us to conclude that these behaviours are regulated by separate transcriptional programs and that melanoma cells, responding to changing microenvironments, can switch between them.
My lab published a molecular taxonomy of melanoma gene expression and used this to construct a gene regulation model for metastatic potential in melanoma (Hoek et al., 2006). This model describes two phenotypic states of melanoma cell and the active signaling pathways responsible for initiating their differences. We successfully tested the capacity for melanoma cells to switch between these states in an in vivo model (Hoek et al., 2008).
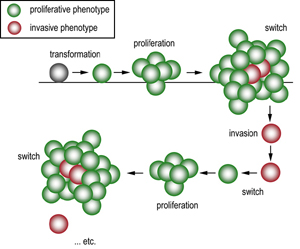 |
| Phenotype switching. After transformation melanoma cells are initially of the proliferative phenotype which promotes tumorigenesis. Changing microenvironmental conditions (such as increasing hypoxia) signals the switch of some cells to an invasive phenotype which escape the primary lesion. These cells lodge elsewhere in the body and switch back to the proliferative phenotype to seed a metastatic lesion, which allows the cycle to be repeated. |
This model implies that metastases are composed of each of two melanoma cell phenotypes and the transition between cell phenotypes (i.e. that cells of one phenotype can give rise to cells of the other) is dominated by microenvironmental conditions and reversible. If this proves to be an accurate description it offers a possible explanation for why monotherapies and current combinatorial therapies fail to control melanoma (for example, chemotherapy targets fast dividing cells, which represent only half of the above model). The strategy should be to first recognize that melanoma is not one disease but two, and in order to be successful a therapy must take them
both into consideration.
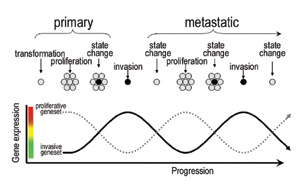 |
| Phenotype switching. Early phase melanoma cells expressing the “proliferative signature” gene set proliferate to form the primary lesion. Following this an unknown signal switch, likely brought about by altered microenvironmental conditions (e.g. hypoxia or inflammation), gives rise to cells with a significantly different “invasive signature” gene set. Invasive signature cells escape and, upon reaching a suitable distal site, revert to the proliferative state and nucleate a new metastasis where the cycle is repeated. Each switch in phenotype (state change) is accompanied by an exchange in expressed gene sets from proliferative to invasive and vice versa. |

